SPOTLIGHT was an open-label, prospective, Phase 3, multicenter, single-dose imaging study investigating the safety and efficacy of POSLUMA PET imaging in 391 men with suspected prostate cancer recurrence.1,2 Please see additional study design below.
COPRIMARY ENDPOINTS
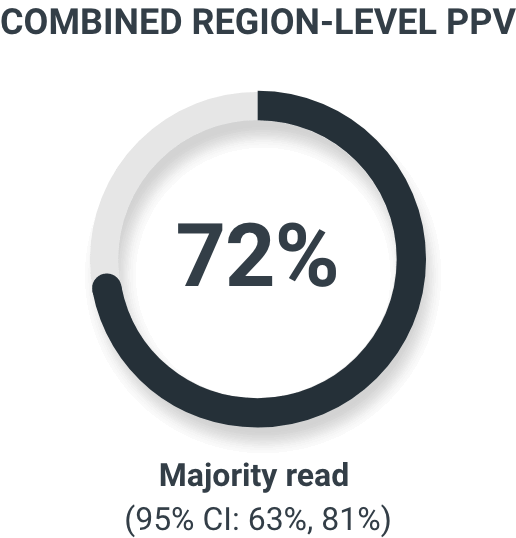
The coprimary endpoints were patient-level verified detection rate (VDR) and combined region-level positive predictive value (PPV) using histopathology or imaging as a standard of truth.2
‡Each patient had a maximum of 3 regions; regions included prostate/prostate bed, pelvic lymph nodes, and other extrapelvic sites, including bone, extrapelvic nodes, viscera, and other soft tissues.
DETECTION AT LOW PSA
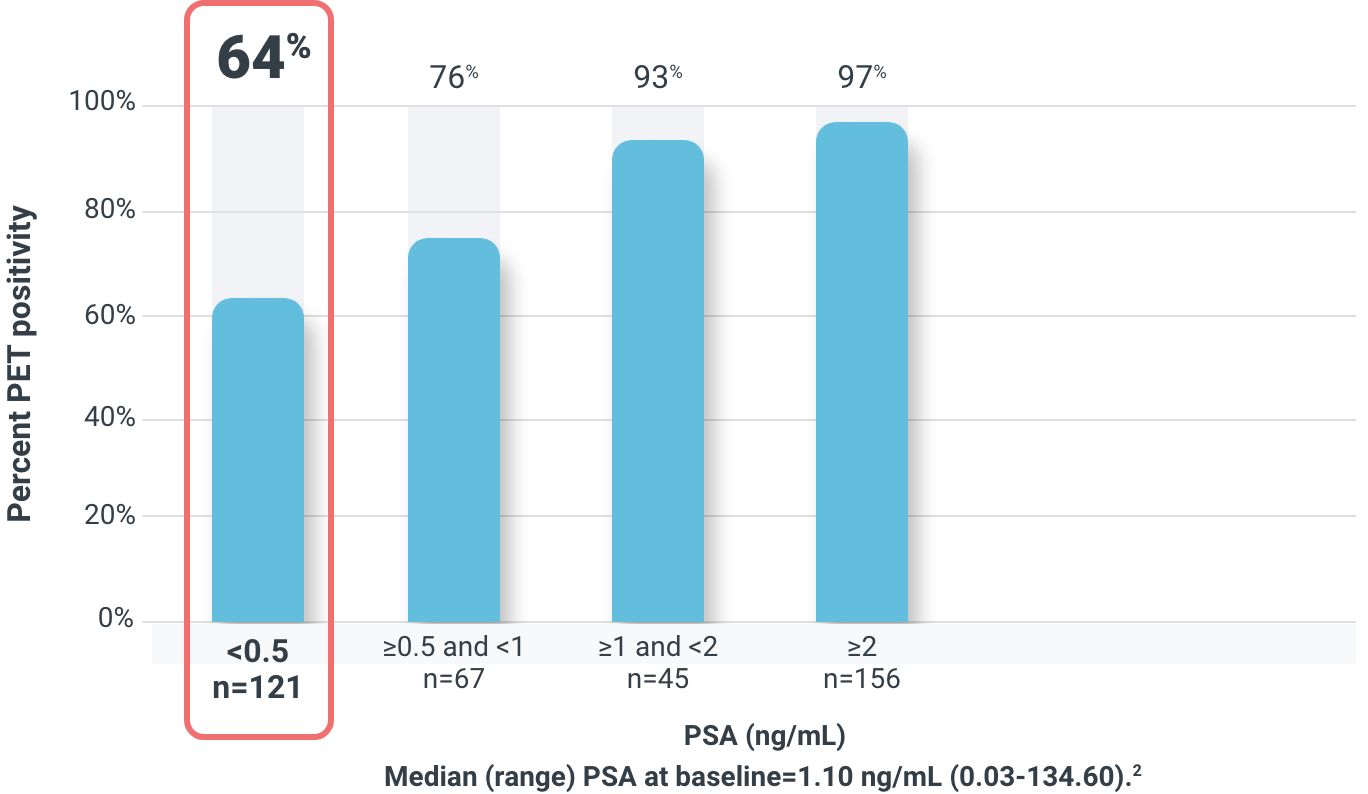
Overall detection rate by majority read was 83% (322/389)1
94% (366/389) of all patients had ≥1 POSLUMA-positive lesion detected by at least 1 reader1
78% (211/269) of patients with negative baseline imaging had POSLUMA-avid lesions2
POSLUMA detected lesions as small as 5 mm3
In patients with an evaluable scan, across 3 readers and compared to reference imaging* or histopathology (N=389)1†
48% to 51%
of patients had 1 or more verified POSLUMA-positive lesions
46% to 60%
of all POSLUMA-positive regions were confirmed true positive
*Imaging reference standard included CT, MRI, Technetium 99m bone scan, or fluciclovine F 18 PET.
†POSLUMA-positive interpretations were compared to the reference standard using a lesion-to-lesion colocalization method and separate consensus panel. Regions include prostate/prostate bed, pelvic lymph nodes, and other.
In patients with histopathology available as the standard of truth (N=69)
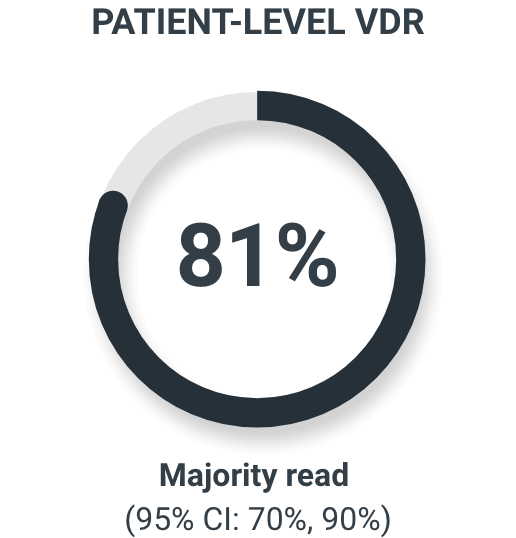
82% majority read patient-level PPV in patients with histopathology as a standard of truth2
CHANGE IN MANAGEMENT
89%
of patients
had a change in intended
treatment (86/97)*
91%
had a major change in treatment (78/86)†
9%
had a minor change in treatment (8/86)‡
*97 out of 391 men had sufficient information to determine change in treatment. Out of the 167 patients with both a pre-PSMA and post-PSMA patient management plan, 70 had either insufficient or invalid information available with which to determine change in treatment planning.
†Major changes included a change from watchful waiting to salvage or noncurative therapy, a change from salvage to noncurative systemic therapy, a change from salvage or noncurative systemic therapy to watchful waiting, or an alternative major change.
‡Minor changes included modified RT field plan, modified ADT regimen, or an alternative minor change.
ADT=androgen deprivation therapy; RT=radiation therapy.

Patient management
Listen to Dr. Sean Collins, a radiation oncologist, explain POSLUMA’s role in patient management.
References: 1. POSLUMA. Package insert. Blue Earth Diagnostics Ltd; 2023. 2. Jani AB, Ravizzini GC, Gartrell BA, et al. Diagnostic performance and safety of 18F-rhPSMA-7.3 PET in men with suspected prostate cancer recurrence: results from a phase 3, prospective, multicenter study (SPOTLIGHT).J Urol. Published online April 26, 2023. doi:10.1097/JU.0000000000003493 3. Data on file. SPOTLIGHT clinical study report. Blue Earth Diagnostics, Ltd. Oxford, UK.